The vanillin to vanillyl alcohol mechanism unveils a captivating journey into the intricacies of organic chemistry, showcasing a transformation that holds immense significance in various industries. This mechanism, characterized by its elegance and versatility, provides a profound understanding of the conversion process and its diverse applications.
Delving into the heart of this mechanism, we unravel the intricate interplay of catalysts, reaction conditions, and the underlying chemical reactions. By dissecting each step, we uncover the factors that govern the efficiency and selectivity of this transformation, paving the way for tailored synthesis strategies.
Overview of Vanillin to Vanillyl Alcohol Mechanism
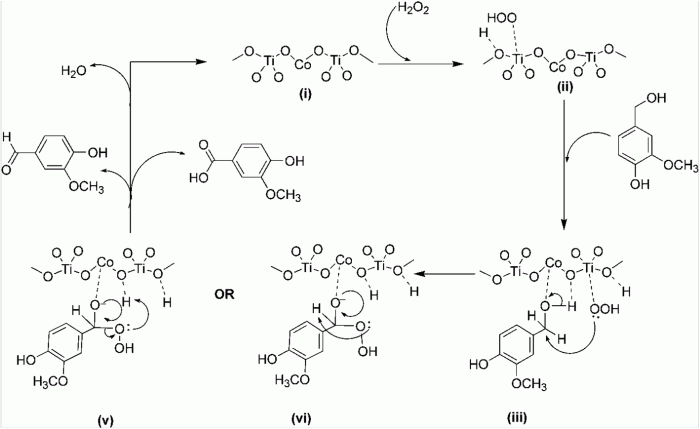
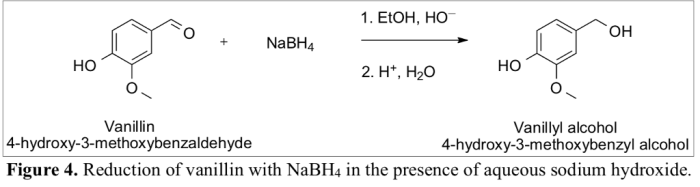
Vanillin, a natural compound found in vanilla beans, is a versatile starting material for the synthesis of various value-added products. One important transformation is the conversion of vanillin to vanillyl alcohol, which is widely used in the pharmaceutical, food, and fragrance industries.
The chemical structure of vanillin is characterized by a phenolic hydroxyl group, an aldehyde group, and a methoxy group. Vanillyl alcohol, on the other hand, has a hydroxyl group in place of the aldehyde group.
The conversion of vanillin to vanillyl alcohol typically involves a reduction reaction, where the aldehyde group is reduced to a hydroxyl group. This reaction can be catalyzed by a variety of reagents, including sodium borohydride, lithium aluminum hydride, and hydrogen gas in the presence of a catalyst.
Role of Catalysts, Vanillin to vanillyl alcohol mechanism
Catalysts play a crucial role in the conversion of vanillin to vanillyl alcohol by enhancing the reaction rate and selectivity. Different catalysts can have varying effects on the reaction outcome.
- Sodium borohydride (NaBH4): A mild reducing agent that selectively reduces the aldehyde group to a hydroxyl group, resulting in high yields of vanillyl alcohol.
- Lithium aluminum hydride (LiAlH4): A stronger reducing agent that can also reduce other functional groups present in vanillin, potentially leading to side reactions and lower selectivity.
- Hydrogen gas (H2): Can be used in the presence of a catalyst, such as palladium or platinum, to reduce the aldehyde group. This method offers high selectivity but may require specialized equipment.
Reaction Conditions
The optimal reaction conditions for the conversion of vanillin to vanillyl alcohol depend on the catalyst used and the desired selectivity. Key factors to consider include:
- Temperature:Typically, the reaction is carried out at room temperature or slightly elevated temperatures (up to 50°C) to minimize side reactions.
- pH:The pH of the reaction medium can affect the activity and selectivity of the catalyst. Neutral or slightly acidic conditions are often preferred.
- Solvent:The choice of solvent can influence the solubility of the reactants and the reaction rate. Common solvents used include methanol, ethanol, and water.
Reaction Mechanism
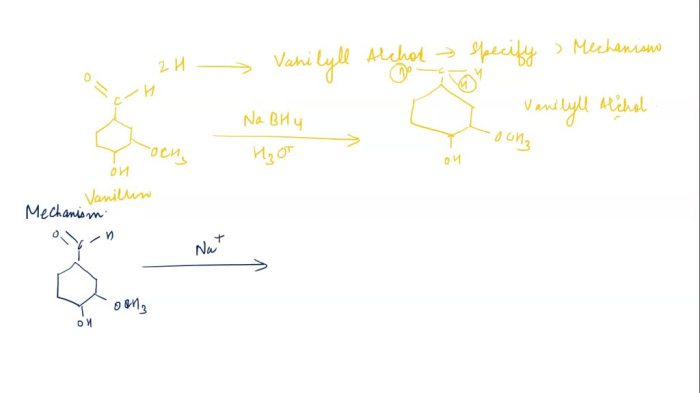
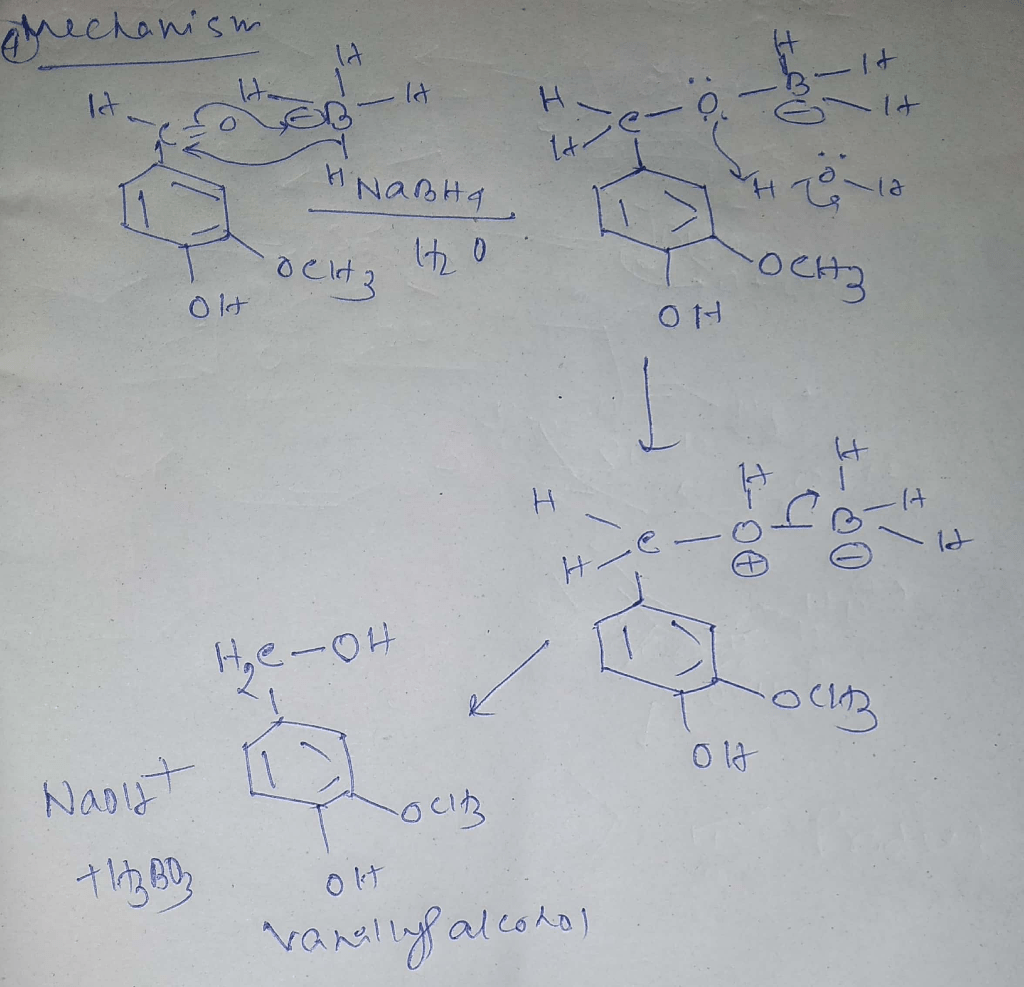
The conversion of vanillin to vanillyl alcohol involves a nucleophilic attack by the hydride ion (H –) on the carbonyl carbon of the aldehyde group. This results in the formation of a tetrahedral intermediate, which subsequently collapses to form vanillyl alcohol and water.
The following chemical equation illustrates the reaction mechanism:
Vanillin + NaBH4→ Vanillyl alcohol + NaBO 2+ H 2O
Applications of Vanillyl Alcohol
Vanillyl alcohol is a valuable intermediate in the synthesis of various compounds, including:
- Pharmaceuticals:Used as an active ingredient in drugs for treating pain, inflammation, and migraines.
- Food additives:Imparts a sweet, vanilla-like flavor to foods and beverages.
- Fragrances:Used as a base note in perfumes and cosmetics, contributing a warm, woody scent.
Question Bank
What is the significance of the vanillin to vanillyl alcohol mechanism?
This mechanism provides a detailed understanding of the conversion process, allowing for the optimization of reaction conditions and the development of efficient synthesis strategies.
How do catalysts influence the vanillin to vanillyl alcohol conversion?
Catalysts play a crucial role in enhancing the reaction rate and selectivity, enabling the efficient production of vanillyl alcohol.
What are the key factors that affect the reaction conditions?
Temperature, pH, and solvent choice are critical factors that influence the efficiency and selectivity of the vanillin to vanillyl alcohol conversion.