Compare and contrast serine proteases and aspartic proteases, delving into the intricacies of their mechanisms, specificities, and physiological roles, unraveling the complexities of these enzymatic powerhouses.
These proteolytic enzymes, each possessing distinct characteristics, play pivotal roles in a myriad of biological processes, shaping the very fabric of life. Their catalytic prowess and substrate preferences set them apart, while their physiological functions and clinical significance intertwine in intricate ways.
Introduction: Compare And Contrast Serine Proteases And Aspartic Proteases
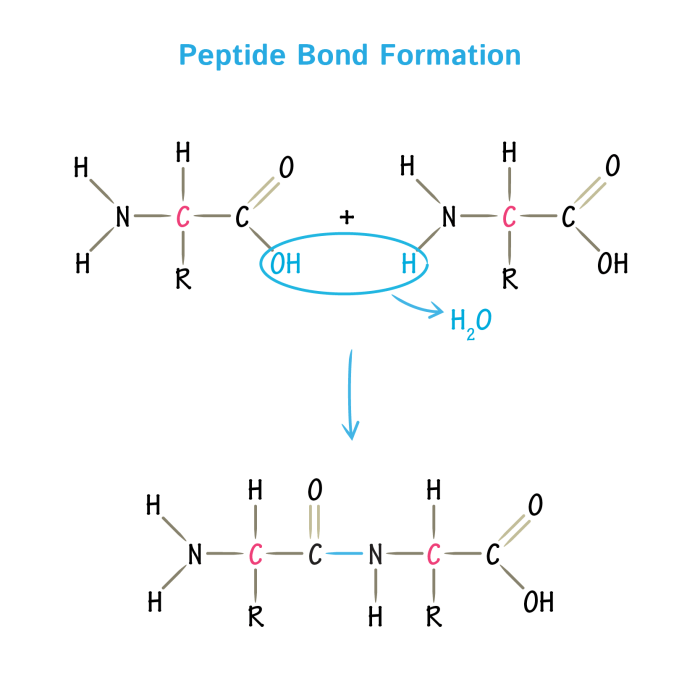
Proteases are enzymes that catalyze the hydrolysis of peptide bonds in proteins. They play crucial roles in a wide range of biological processes, including digestion, blood coagulation, and immune responses.
Serine proteases and aspartic proteases are two major classes of proteases that differ in their catalytic mechanisms and substrate specificities.
Mechanism of Action
Serine Proteases
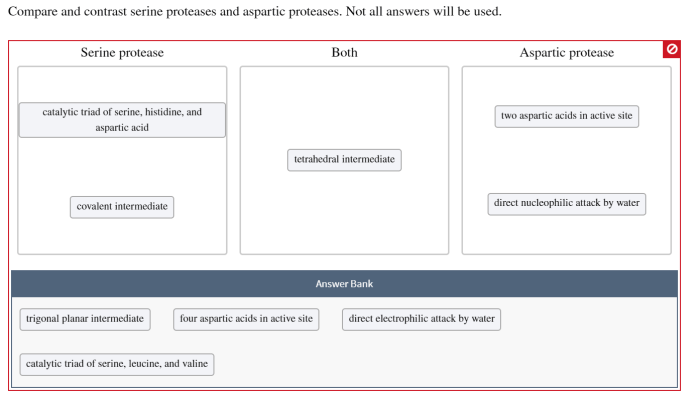
Serine proteases utilize a catalytic triad consisting of serine, histidine, and aspartate residues. The serine residue acts as the nucleophile, attacking the peptide bond, while the histidine and aspartate residues facilitate proton transfer and stabilize the transition state.
Aspartic Proteases
Aspartic proteases employ two aspartic acid residues in their catalytic mechanism. One aspartic acid acts as the nucleophile, while the other stabilizes the transition state through hydrogen bonding. The presence of two aspartic acid residues results in a highly acidic active site.
Comparison
Both serine and aspartic proteases hydrolyze peptide bonds through a nucleophilic attack mechanism. However, they differ in the nature of their catalytic residues and the pH optimum of their activity.
Substrate Specificity
Serine Proteases
Serine proteases exhibit a broad substrate specificity, cleaving a wide range of peptide bonds. The S1 pocket, which accommodates the residue immediately preceding the scissile bond, plays a critical role in substrate recognition.
Aspartic Proteases
Aspartic proteases have a more restricted substrate specificity, primarily cleaving peptide bonds with acidic residues in the S1′ position (the residue following the scissile bond). This specificity is due to the presence of a negatively charged aspartate residue in the S1′ pocket.
Comparison
Serine proteases possess a broader substrate specificity compared to aspartic proteases. The S1 and S1′ pockets of serine and aspartic proteases, respectively, determine their substrate preferences.
Inhibitors
Serine Protease Inhibitors, Compare and contrast serine proteases and aspartic proteases
Serine protease inhibitors (serpins) are a class of proteins that regulate serine protease activity. They form a complex with the protease, leading to a conformational change that traps the protease in an inactive state.
Aspartic Protease Inhibitors
Aspartic protease inhibitors, such as pepstatin, bind to the active site of aspartic proteases and prevent substrate binding. They form a complex that blocks the access of substrates to the catalytic site.
Comparison
Both serine and aspartic protease inhibitors are effective in regulating protease activity. However, they differ in their mechanisms of action and target specificity.
Physiological Roles
Serine Proteases
- Digestion: Trypsin, chymotrypsin, and elastase are serine proteases involved in the digestion of proteins in the gastrointestinal tract.
- Blood coagulation: Thrombin is a serine protease that plays a crucial role in blood clotting by converting fibrinogen to fibrin.
- Immune responses: Granzymes are serine proteases released by cytotoxic T cells and natural killer cells to induce apoptosis in target cells.
Aspartic Proteases
- HIV-1 protease: This aspartic protease is essential for the replication of the HIV-1 virus, cleaving viral polyproteins into functional subunits.
- Renin: An aspartic protease involved in the regulation of blood pressure by converting angiotensinogen to angiotensin I.
- Cathepsins: Aspartic proteases found in lysosomes, involved in protein degradation and cellular processes.
Comparison
Serine and aspartic proteases play diverse physiological roles in various biological processes, including digestion, blood coagulation, immune responses, and viral replication.
Clinical Significance
Serine Proteases
- Cancer: Serine proteases are involved in tumor growth, invasion, and metastasis. Dysregulation of serine protease activity can contribute to cancer development and progression.
- Inflammation: Serine proteases are key mediators of inflammation, promoting the recruitment of immune cells and tissue remodeling.
Aspartic Proteases
- HIV infection: HIV-1 protease is a target for antiviral therapy, as its inhibition prevents viral replication and reduces disease progression.
- Alzheimer’s disease: Aspartic proteases, such as beta-secretase, are involved in the processing of amyloid precursor protein, leading to the formation of amyloid plaques in Alzheimer’s disease.
Comparison
Serine and aspartic proteases have significant clinical implications in various diseases, including cancer, inflammation, HIV infection, and neurodegenerative disorders.
Expert Answers
What is the key difference between serine and aspartic proteases?
Serine proteases utilize a catalytic triad involving serine, histidine, and aspartic acid, while aspartic proteases employ two aspartic acid residues for catalysis.
How do serine proteases exhibit substrate specificity?
The S1 pocket of serine proteases accommodates specific amino acid side chains, determining their substrate preferences.
What are some examples of serine proteases and their physiological roles?
Trypsin aids in digestion, thrombin promotes blood clotting, and kallikreins participate in inflammation.
How do aspartic protease inhibitors contribute to drug development?
Aspartic protease inhibitors, such as pepstatin, serve as potential therapeutic agents for treating HIV and Alzheimer’s disease.