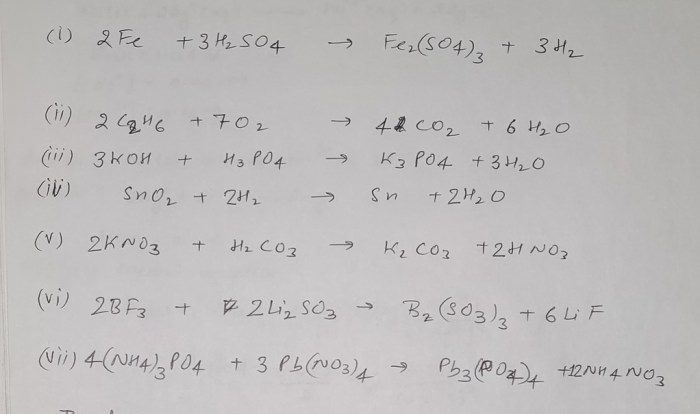Embark on a comprehensive exploration of matter classification, unraveling the fundamental principles that govern the nature and behavior of substances. Delving into the classification of matter answer key pogil, this discourse elucidates the distinct states of matter, their characteristic properties, and the factors influencing phase transitions.
Moreover, it delves into the physical and chemical properties that define substances, enabling their identification and classification. By understanding the intricacies of matter classification, we gain a deeper appreciation for the diverse materials that shape our world and the practical applications that stem from this knowledge.
The subsequent paragraphs provide a detailed examination of the physical properties of matter, such as density, solubility, and conductivity, and explore how these properties are harnessed to identify and differentiate substances. The discussion extends to chemical properties, encompassing combustion, oxidation, and neutralization reactions, and their significance in classifying substances.
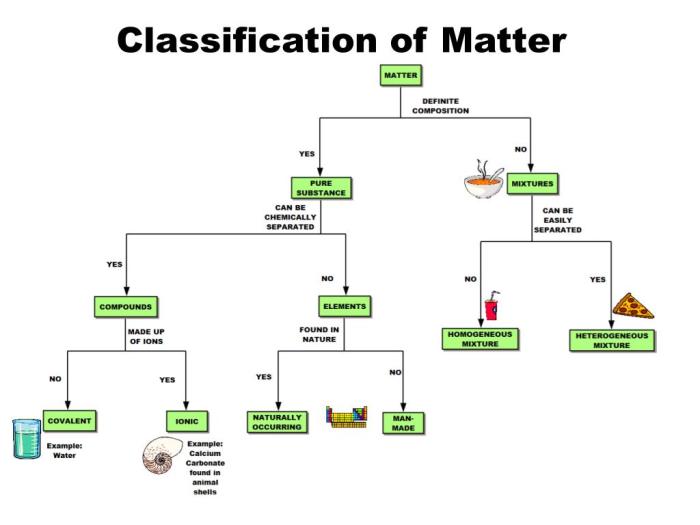
Furthermore, the distinction between mixtures and compounds is clarified, along with methods for separating mixtures and examples of common mixtures and compounds.
Classification of Matter: Key Concepts: Classification Of Matter Answer Key Pogil
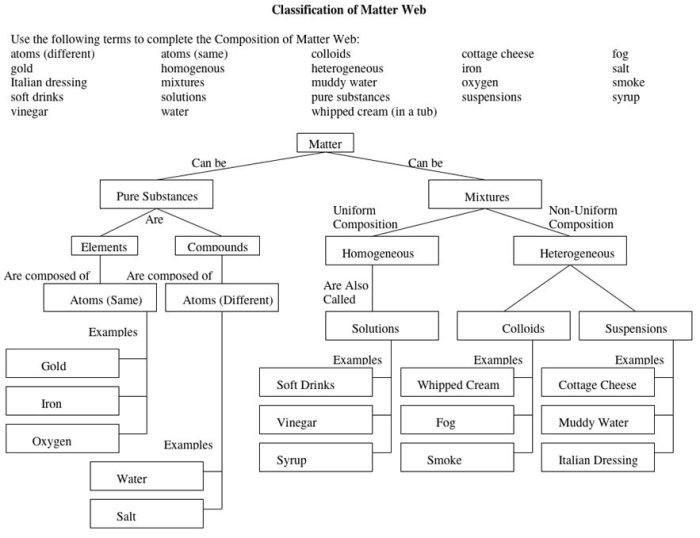
Matter, the physical substance that makes up everything in the universe, can be classified into different states based on its properties and behavior. The three main states of matter are solids, liquids, and gases. Solids have a definite shape and volume, liquids have a definite volume but no definite shape, and gases have no definite shape or volume.
These states can transition into each other through phase transitions, which are influenced by factors such as temperature and pressure.
Physical Properties of Matter
Physical properties are characteristics of matter that can be observed or measured without changing its chemical composition. These properties include density, solubility, and conductivity. Density is the mass per unit volume of a substance, solubility is its ability to dissolve in a solvent, and conductivity is its ability to conduct heat or electricity.
These properties can be used to identify and classify substances, and they have practical applications in various fields.
Chemical Properties of Matter
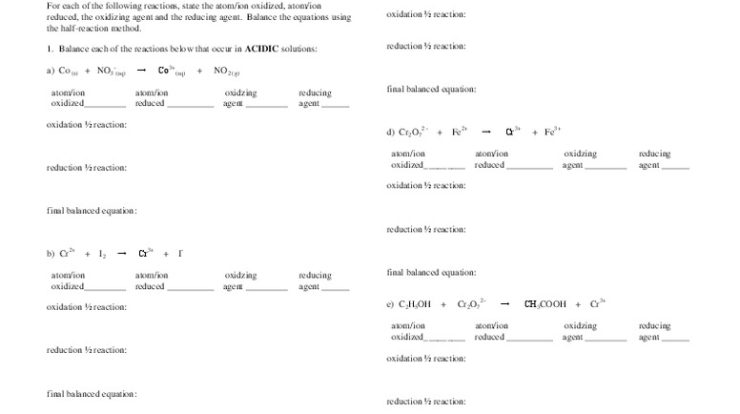
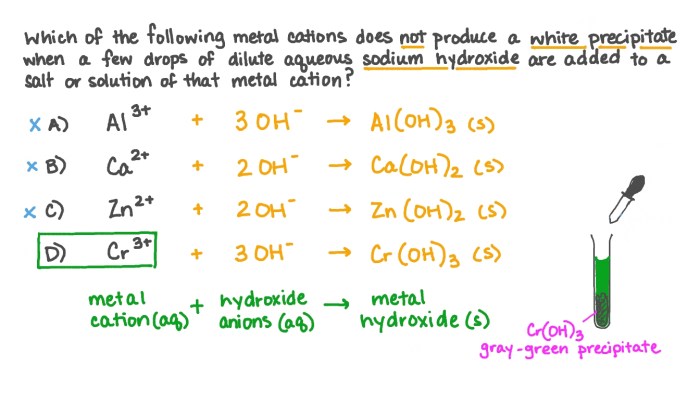
Chemical properties describe how substances react with other substances to form new substances. These properties include flammability, reactivity with acids or bases, and oxidation resistance. Chemical reactions involve changes in the chemical composition of the substances involved, and they can be classified into different types, such as combustion, oxidation, and neutralization.
Understanding chemical properties is crucial for predicting and controlling chemical reactions.
Mixtures and Compounds, Classification of matter answer key pogil
Mixtures are combinations of two or more substances that retain their individual chemical identities. They can be separated into their components through physical methods like filtration, distillation, or chromatography. Compounds, on the other hand, are substances composed of two or more elements chemically combined in fixed proportions.
They have unique properties different from their constituent elements and cannot be separated into simpler substances by physical means.
Applications of Matter Classification
The classification of matter has wide-ranging applications in various fields. In chemistry, it helps identify and characterize substances, predict their behavior, and design new materials. In materials science, it guides the development of materials with specific properties for different applications.
In environmental science, it aids in understanding the behavior of pollutants and designing strategies for pollution control.
FAQs
What are the three states of matter?
Solid, liquid, and gas
What is the difference between a physical and chemical property?
Physical properties can be observed without changing the composition of the substance, while chemical properties describe the substance’s ability to undergo chemical reactions.
What is the difference between a mixture and a compound?
A mixture is a combination of two or more substances that retain their individual identities, while a compound is a new substance formed by the chemical combination of two or more elements.