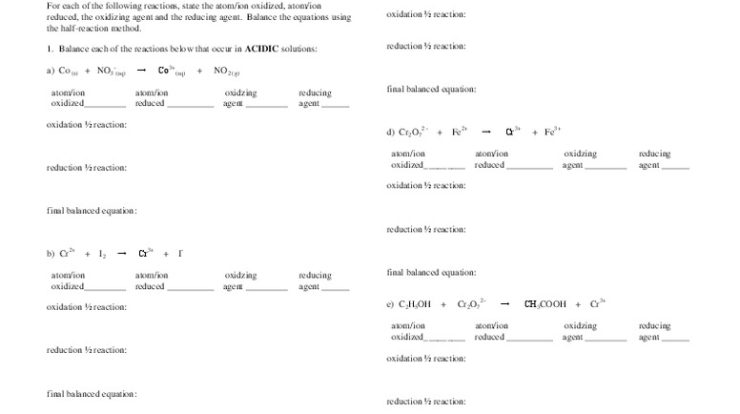
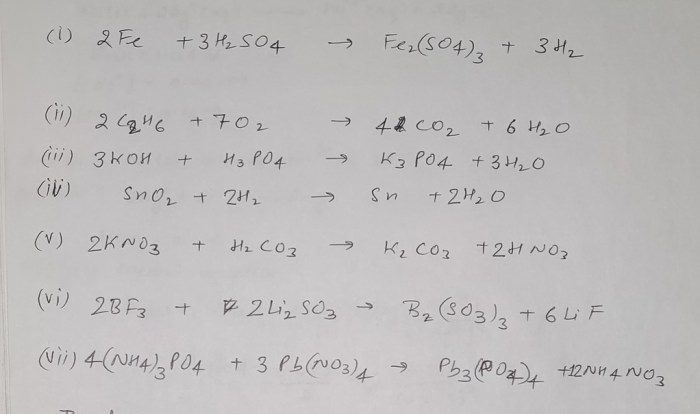M+ is an unknown metal cation – m+, an enigmatic metal cation, beckons us into a realm of chemical intrigue. Encountered in diverse settings, from the depths of the ocean to the intricacies of industrial processes, this unknown entity demands our attention.
Identifying m+ is not merely an academic pursuit; it holds practical significance, unlocking doors to environmental monitoring, forensic investigations, and materials science advancements.
Introduction to Unknown Metal Cation
An unknown metal cation is a metal ion whose identity is not immediately known. This can occur in various scenarios, such as during chemical analysis, environmental monitoring, or forensic investigations. Identifying unknown metal cations is crucial for understanding their potential effects on human health and the environment, as well as for determining appropriate remediation strategies.
Methods for Identifying Unknown Metal Cations: M+ Is An Unknown Metal Cation

Identifying unknown metal cations is crucial in various fields, including analytical chemistry, environmental monitoring, and forensic science. Several analytical techniques are employed for this purpose, each with its advantages and limitations.
The choice of technique depends on factors such as the sample’s complexity, the cation’s concentration, and the available instrumentation.
Spectroscopic Techniques
Spectroscopic techniques involve analyzing the interaction of light with the sample. These techniques include:
- Atomic Absorption Spectroscopy (AAS):AAS measures the absorption of light by metal ions at specific wavelengths. It is a sensitive and selective technique suitable for analyzing trace metal concentrations.
- Flame Emission Spectroscopy (FES):FES measures the emission of light by excited metal ions. It is less sensitive than AAS but can provide qualitative information about the presence of certain metals.
Electrochemical Techniques
Electrochemical techniques involve measuring the electrical properties of the sample. These techniques include:
- Potentiometry:Potentiometry measures the potential difference between a reference electrode and an indicator electrode immersed in the sample. It is used to determine the concentration of metal ions that undergo redox reactions.
- Voltammetry:Voltammetry applies a voltage to the sample and measures the resulting current. It can provide information about the oxidation-reduction behavior of metal ions.
Chromatographic Techniques, M+ is an unknown metal cation
Chromatographic techniques separate metal ions based on their different physical or chemical properties. These techniques include:
- Ion Chromatography (IC):IC separates metal ions based on their charge and size. It is used to analyze complex samples containing multiple metal ions.
- High-Performance Liquid Chromatography (HPLC):HPLC separates metal ions based on their interactions with a stationary phase. It can be used to analyze both inorganic and organic metal complexes.
Other Techniques
Other techniques used for identifying unknown metal cations include:
- X-ray Fluorescence (XRF):XRF exposes the sample to X-rays and measures the emitted fluorescent radiation. It is a non-destructive technique that can provide qualitative and quantitative information about metal ions.
- Inductively Coupled Plasma Mass Spectrometry (ICP-MS):ICP-MS uses an inductively coupled plasma to ionize metal ions and measures their mass-to-charge ratio. It is a highly sensitive and versatile technique that can analyze a wide range of metal ions.
| Technique | Advantages | Disadvantages |
|---|---|---|
| AAS | – High sensitivity
|
– Limited to metals that absorb light
|
| FES | – Inexpensive
|
– Low sensitivity
|
| Potentiometry | – Direct measurement of ion concentration
|
– Limited to redox-active ions
|
| Voltammetry | – Provides information about redox behavior
|
– Requires specialized instrumentation
|
| IC | – Separates ions based on charge and size
Although m+ is an unknown metal cation, it has been extensively studied due to its potential applications in various fields. For example, e flat concert scale clarinet manufacturers use m+ to enhance the instrument’s resonance and tone quality. Moreover, m+ has been explored as a promising catalyst in chemical reactions, showcasing its versatility and potential for further research.
|
– Not as sensitive as other techniques
|
| HPLC | – Separates both inorganic and organic metal complexes
|
– Requires specialized equipment
|
| XRF | – Non-destructive
|
– Not as sensitive as other techniques
|
| ICP-MS | – Highly sensitive
|
– Requires specialized equipment
|
Chemical Reactions of Unknown Metal Cations
Chemical reactions play a crucial role in identifying unknown metal cations. These reactions involve interactions between the metal cation and various reagents, resulting in observable changes that can be used to differentiate between different metal ions.
The chemical reactions of unknown metal cations can be categorized into several types, each providing specific information about the metal ion’s identity:
Precipitation Reactions
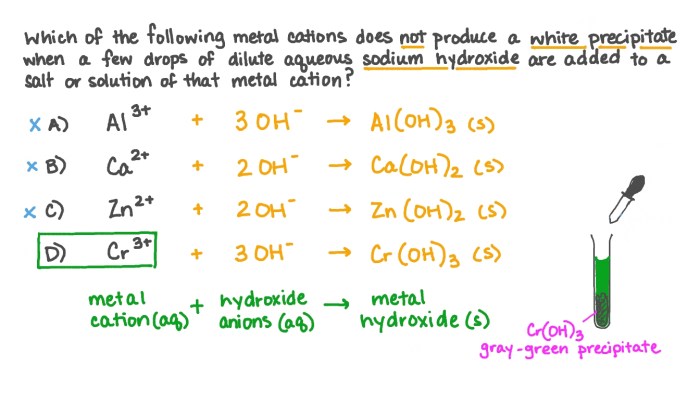
Precipitation reactions occur when a metal cation reacts with a reagent to form an insoluble solid precipitate. The color, texture, and solubility of the precipitate can provide clues about the identity of the metal ion.
- For example, adding sodium hydroxide (NaOH) to a solution containing copper(II) ions (Cu 2+) results in the formation of a blue-green precipitate of copper(II) hydroxide [Cu(OH) 2].
- Similarly, adding barium chloride (BaCl 2) to a solution containing sulfate ions (SO 42-) forms a white precipitate of barium sulfate (BaSO 4).
Complex Formation Reactions
Complex formation reactions involve the formation of a complex ion between a metal cation and a ligand. Ligands are molecules or ions that can donate electron pairs to the metal ion, forming a coordinate bond.
- For example, adding ammonia (NH 3) to a solution containing copper(II) ions (Cu 2+) forms a deep blue complex ion [Cu(NH 3) 4] 2+.
- The formation of colored complex ions can be used to identify metal ions, as different metal ions form complexes with characteristic colors.
Redox Reactions
Redox reactions involve the transfer of electrons between metal ions and other species. These reactions can be used to determine the oxidation state of a metal ion and to differentiate between metal ions with different oxidation states.
- For example, adding potassium permanganate (KMnO 4) to a solution containing iron(II) ions (Fe 2+) results in the oxidation of Fe 2+to Fe 3+and the reduction of MnO 4–to Mn 2+, resulting in a color change from purple to colorless.
- Redox reactions can also be used to separate metal ions based on their reduction potentials.
By carefully observing the chemical reactions of unknown metal cations and interpreting the results, it is possible to identify the metal ions present in a solution and gain valuable information about their chemical properties.
Applications of Unknown Metal Cation Analysis
The analysis of unknown metal cations finds practical applications in numerous fields, providing valuable information for environmental monitoring, forensic science, and materials science. This analysis enables the identification and quantification of metal ions in various samples, allowing scientists and professionals to assess their presence, concentration, and potential impact.
In environmental monitoring, unknown metal cation analysis plays a crucial role in assessing water quality, soil contamination, and air pollution. By analyzing the metal content in environmental samples, researchers can determine the sources and extent of metal contamination, monitor its impact on ecosystems, and develop strategies to mitigate its effects.
Forensic Science
In forensic science, unknown metal cation analysis is employed to analyze trace evidence, such as gunshot residue, paint chips, and metal fragments. By identifying the specific metal ions present in these samples, forensic scientists can link suspects to crime scenes, determine the type of weapon used, and establish the sequence of events.
Materials Science
In materials science, unknown metal cation analysis is utilized to characterize the composition and properties of materials, such as alloys, ceramics, and semiconductors. By identifying the metal ions present and their concentrations, researchers can optimize material properties, develop new alloys with enhanced performance, and investigate the corrosion and degradation mechanisms of materials.
FAQ Explained
What is an unknown metal cation?
An unknown metal cation is a positively charged metal ion whose identity is yet to be determined.
How can we identify unknown metal cations?
Various analytical techniques, such as atomic absorption spectroscopy and ion chromatography, are employed to identify unknown metal cations.
What are the applications of unknown metal cation analysis?
Unknown metal cation analysis finds applications in environmental monitoring, forensic science, and materials science, among other fields.